Miniaturization of an Epigenetic AlphaLISA Assay with the Echo Liquid Handler and the BMG LABTECH PHERAstar FS
Bonnie Edwards1 , Jing Wang1 , Carl Peters2
1Beckman Coulter Life Sciences, 2BMG LABTECH
Abstract
Epigenetics continues to emerge as an important target class for drug discovery and cancer research. As programs scale to evaluate many new targets related to epigenetic expression, new tools and techniques are required to enable efficient and reproducible high-throughput epigenetic screening. The combination of robust and precise instrumentation from Beckman Coulter Life Sciences and BMG LABTECH enable the miniaturization and automation of PerkinElmer AlphaLISA epigenetic assay kits without compromising performance and data quality.
Background
Acoustic Dispensing and the Echo Liquid Handler
The Echo Liquid Handler revolutionizes liquid transfer by using acoustic energy to eject fluids. Echo Liquid Handlers can transfer compounds, samples, reagents, and beads in sub-microliter volumes to high-density assay formats using only acoustic energy – no contact or tips required, thereby reducing the risk of reagent carryover and eliminating tip costs.
Assay miniaturization increases screening throughput and reduces operating costs. With the ability to transfer fluids accurately and precisely in 2.5 nL and 25 nL increments, Echo Liquid Handlers provide a high-throughput liquid handling solution that enables miniaturization of screening assays without compromising data quality.
The PHERAstar FS Plate Reader
The PHERAstar FS multi-mode plate reader, with the highest sensitivity and lowest read time of assays in densities as high as 3456 wells, is a perfect complement to the Echo Liquid Handler. The PHERAstar FS plate reader uses a dedicated laser and assay specific modules for AlphaScreen / AlphaLISA detection that enables data collection at low assay volumes. Together the PHERAstar FS and Echo Liquid Handler offer an unparalleled solution for cost-effective, high-throughput epigenetic screening.
PerkinElmer AlphaLISA Assays
AlphaLISA assays are typically performed at volumes of about 20 to 25 μL. However, with the 2.5 nL and 25 nL dispense increment of Echo Liquid Handlers, assay volumes can be reduced significantly. In this study, we were able to reduce a methyltransferase assay volume to as low as a 5 μL with excellent results.
Experiment
AlphaLISA Methlytransferase Assay
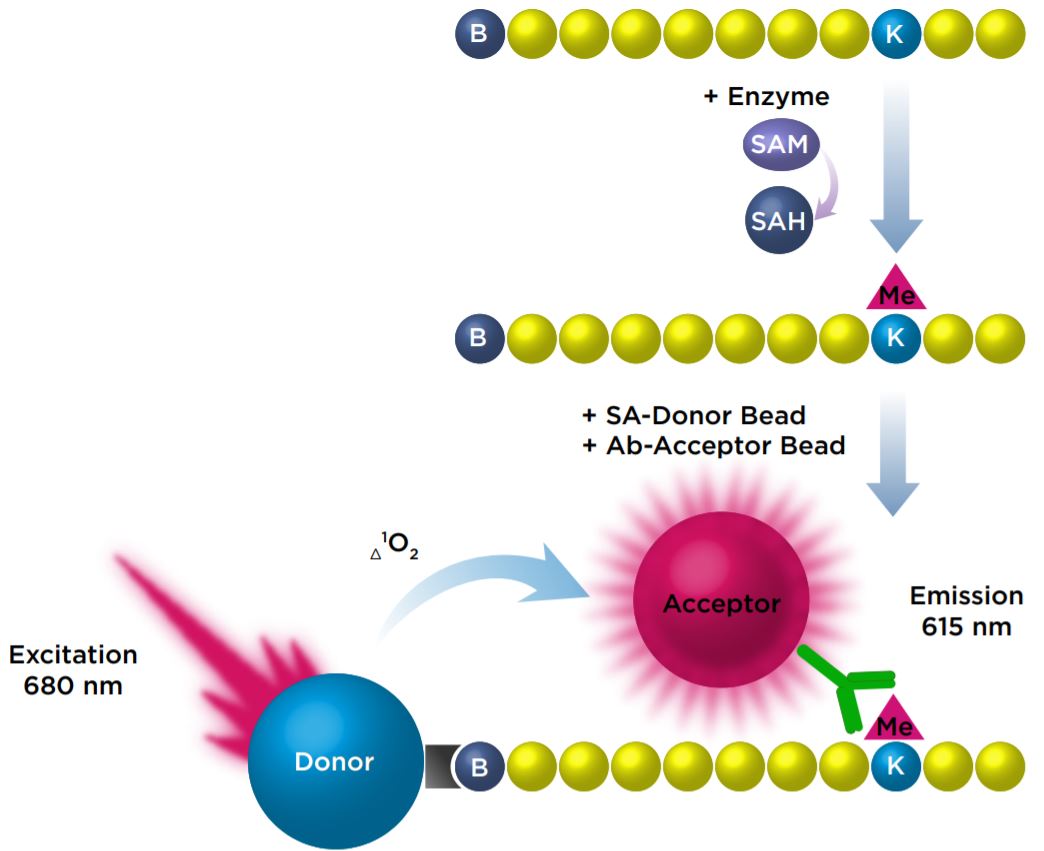
We investigated the miniaturization of an AlphaLISA assay that measured the monomethylation of a biotinylated Histone H3 (1-21) peptide at Lysine 4 with the SET7/9 enzyme. In this assay, antimethyl-Histone H3 Lysine 4 conjugated Acceptor beads bind specifically to the modified Histone H3 peptide. The Streptavidin Donor beads then bind to the biotinylated peptide, which brings them into close proximity to the bound Acceptor beads. Upon excitation of the Donor beads, singlet oxygen molecules are produced, resulting in a transfer of energy to the Acceptor beads, which then emit a signal at 615 nm.
Figure 1: Detection of monomethylation of a peptide with AlphaLISA beads
Methods
A stock solution of S-(5’-adenosyl)-L-methionine chloride (SAM) was prepared at 30 mM in 5 mM H2SO4/10% ethanol (v/v) in H2O. Histone H3 peptide was diluted in assay buffer to a concentration of 200 nM (4X), SAM to 400 nM (4X), and SET7/9 enzyme to 4 nM (4X) just prior to use.
For both the 384-well and 1536-well assays, a dose response curve of two known inhibitors, S-(5’- adenosyl)-L-homocysteine (SAH) and sinefungin, were first added to a 384-well Echo Qualified Microplate source, and then transferred to the assay plates using the Echo 555 Liquid Handler. 40 nL of compound were transferred in the 10 μL assay and 20 nL of compound were transferred in the 5 μL assays.
Assay buffer, enzyme, and peptide + SAM (or peptide only for negative controls) were then added to the plates. In the 5 μL and 10 μL assays, the additions were performed using the Echo 525 Liquid Handler. The plates were sealed, briefly centrifuged at 1000 rpm, and incubated for 30 minutes at room temperature (RT).
Anti-methyl-Histone H3 Lysine 4 (H3K4me1-2) AlphaLISA Acceptor beads were diluted in 1X Epigenetics Buffer 1 at a concentration of 100 μg/mL, then added to a 384-well Echo Qualified Microplate source. Beads were then transferred to the assay plates with the Echo 525 Liquid Handler to a final concentration of 20 μg/mL. Plates were re-sealed, briefly centrifuged at 1000 rpm, shaken, and incubated for 60 minutes at RT, in the dark.
Streptavidin Donor beads were diluted in 1X Epigenetics Buffer 1 at a concentration of 50 μg/mL, added to a 384-well Echo Qualified Microplate source, and transferred to the assay plates with the Echo 525 Liquid Handler to a final concentration of 20 μg/mL. Plates were re-sealed, briefly centrifuged at 1000 rpm, shaken, and incubated for 30 minutes at RT, in the dark. Plates were then read on the PHERAstar FS plate reader.
As a comparison of signal to background (S/B) and Z’ factors, positive and negative controls were also run at the standard assay volume of 25 μL in 384-well format. Reagent additions were done with manual pipetting.
| Total Volume | 25 µL | 10 µL | 5 µL |
| Assay Buffer | 5 µL | 2 μL | 1 μL |
| Compound or DMSO | 100 nL | 40 nL | 20 nL |
| SET7/9 Enzyme (4X) | 2.5 μL | 1 μL | 500 nL |
| H3/SAM Mix (4X) | 2.5 μL | 1 μL | 500 nL |
| Alpha Acceptor Beads | 5 μL | 2 μL | 1 μL |
| Alpha Donor Beads | 10 μL | 4 μL | 2 μL |
Table 1: Volumes of reagents and sample added for each assay total volume
Methods were adapted from Gauthier, N, et al., Development of Homogeneous Nonradioactive Methyltransferase and Demethylase Assays Targeting Histone H3 Lysine 4, J Biomol Screen, 2012 17:49, online 21 September 2011
Results
We calculated signal to background and Z’ factors for the different assay formats and compared the data for the miniaturized assay formats to the 25 μL assay format. While signal to background was lower in the miniaturized assays, overall assay quality in the miniaturized assay formats was still excellent, with Z’ factors of 0.82 and above for all formats. In comparison, the 25 μL assay, which was assembled manually, had a Z’ factor of 0.73.
| Assay Format | Dispense Technique | S/B | Z' Factor |
| 384-well, 25 μL | Manual | 9.4 | 0.73 |
| 384-well, 10 μL | Echo System | 6.6 | 0.82 |
| 384-well, 5 μL | Echo System | 5.5 | 0.83 |
| 1536-well, 1 μL | Echo System | 6.5 | 0.85 |
Table 2: Comparison of signal-to-background and Z’ factors between standard volume assay and miniaturized assay formats
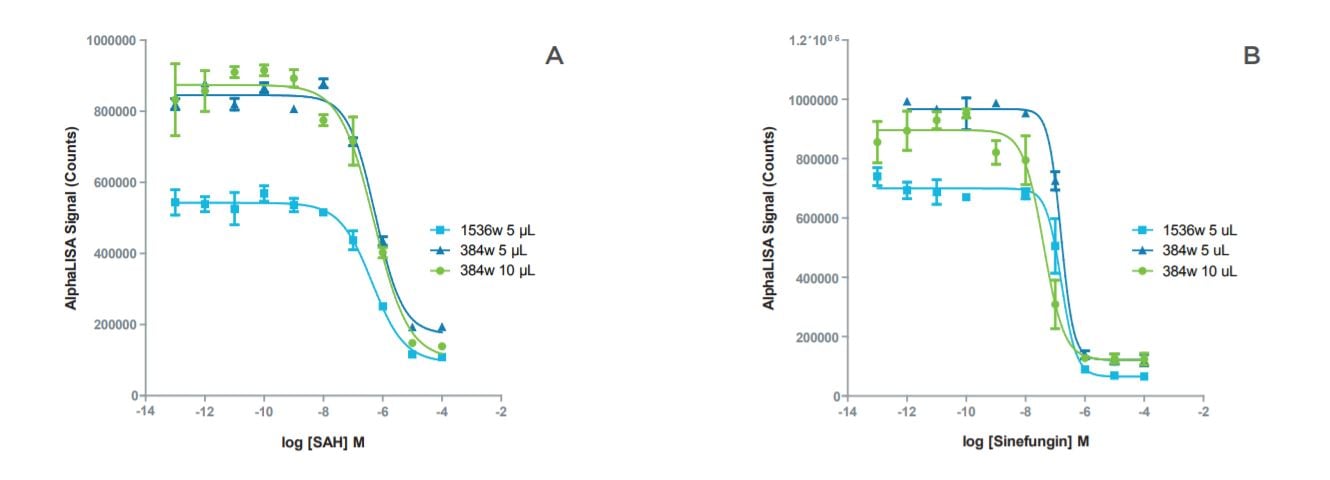
Figure 2: Inhibition curves for SAH and sinefungin showed good agreement in IC50 values between the miniaturized formats. A: Inhibition curves for SAH in miniaturized assay formats. B: Inhibition curves for sinefungin in miniaturized assay formats.
| Assay Format | IC50 - SAH | IC50 - Sinefungin |
| 384-well, 10 μL | 515 nM | 40 mM |
| 384-well, 5 μL | 566 nM | 156 nM |
| 1536-well, 5 μL | 441 nM | 161 nM |
Table 3: Comparison of IC50 values for SAH and sinefungin in standard volume and miniaturized assay formats
Automation with the Access Laboratory Workstation
An epigenetic AlphaLISA assay can be readily adapted to automation with the Access Laboratory Workstation. The Access system allows seamless integration of an Echo Liquid Handler, including bulk fillers, plate sealers, plate peelers, centrifuges, and plate readers such as the PHERAstar FS. Tempo Automation Control Software manages all plate processing and has scheduling features to control plate incubation times. In this way, assay assembly can be automated from start to read on the Access system, thereby increasing throughput and maximizing walk-away time.
Figure 3: Access Laboratory Workstation featuring an Echo Liquid Handler and PHERAstar FS plate reader
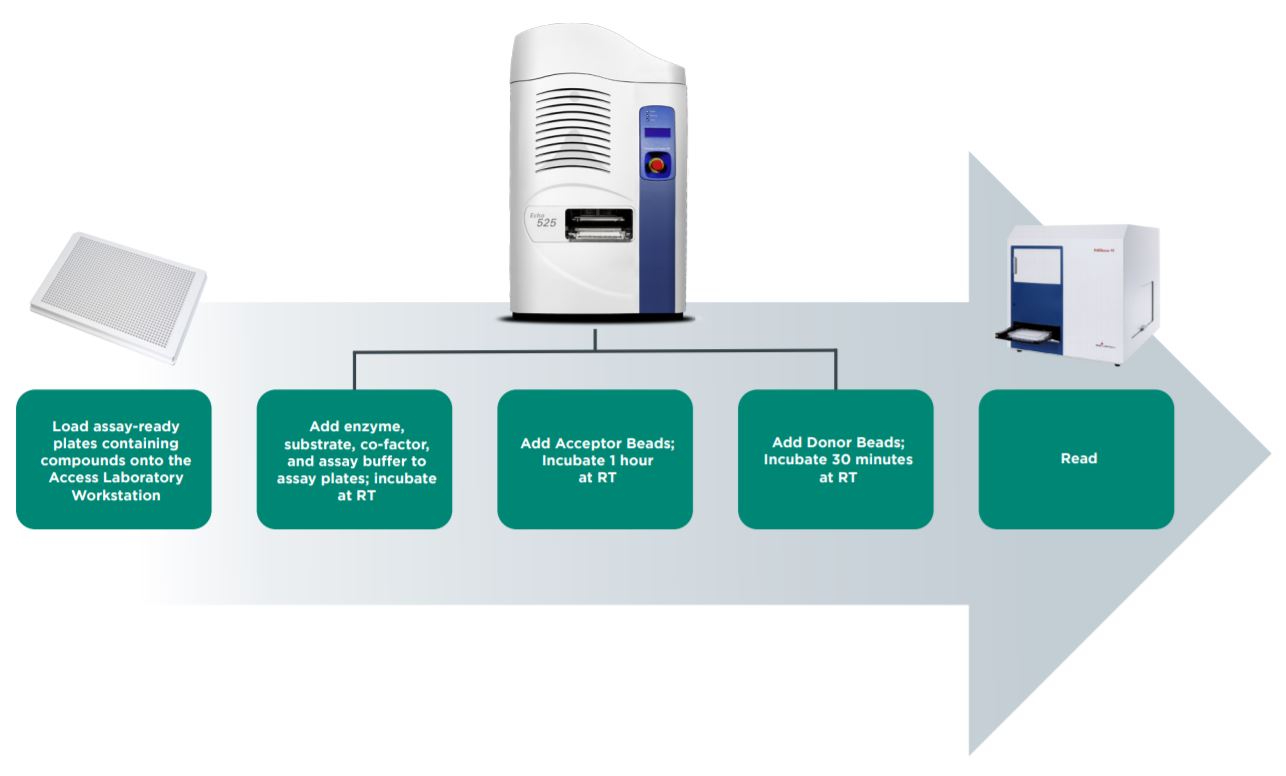
Figure 4: Automated workflow for AlphaLISA epigenetic assay
Summary
As epigenetics continues to grow as an area of focus, there is a greater need to be able to run these assays on a larger scale in a cost-effective manner. Doing so requires the ability to adapt these assays to miniaturization and higher-density formats. With the Echo Liquid Handler, reagents, and compounds can be dispensed accurately and precisely in nanoliter volumes, enabling the assembly of miniaturized AlphaLISA epigenetic assays while maintaining or improving data quality. Additionally, with the Access Laboratory Workstation, the entire workflow can be easily automated to increase throughput and productivity.
Materials
| Equipment | Manufacturer |
| Echo 525 Liquid Handler | Beckman Coulter Life Sciences |
| Echo 555 Liquid Handler | Beckman Coulter Life Sciences |
| Microplate Centrifuge | Agilent Technologies |
| PlateLoc Thermal Microplate Sealer | Agilent Technologies |
| PHERAstar FS Plate Reader | BMG LABTECH |
| XPeel Automated Microplate Seal Removal | Brooks Automation |
| Teleshake | INHECO |
| Reagents | Manufacturer | Part Number |
| SET7/9 (human) Enzyme, Recombinant | Enzo Life Sciences | ALX-201-178-C100 |
| Histone H3 (1 - 21) Peptide, Biotinylated | AnaSpec | AS-61702 |
| Anti-methyl-Histone H3 Lysine 4 (H3K4me1-2) AlphaLISA Acceptor Beads | PerkinElmer | AL116 |
| Alpha Streptavidin Donor Beads | PerkinElmer | 6760002 |
| AlphaLISA 5X Epigenetics Buffer 1 Kit | PerkinElmer | AL008 |
| S-(5’-Adenosyl)-L-methionine chloride (SAM) | Sigma | A7007 |
| S-(5’-Adenosyl)-L-homocysteine (SAH) | Sigma | A9384 |
| Sinefungin | Enzo Life Sciences | ALX-380-070 |
Assay buffer: 50 mM Tris-HCl, pH 8.8, 5 mM MgCl2, 1 mM DTT, 0.01% Tween-20
| Consumables | Manufacturer | Part Number |
| 384-well Polypropylene Echo Qualified Microplate | Beckman Coulter Life Sciences | P-05525 |
| 384-well Round Bottom, White Microplate | Corning Life Sciences | 3674 |
| 1536-well Flat Bottom, White Microplate | Corning Life Sciences | 3729 |