Error Prevention Features of the AQUIOS CL Overview
Introduction
Laboratories follow procedures defined by laboratory standards and regulations set forth by regulatory bodies such as the Centers for Disease Control (CDC) among others. The procedures are in place as safety countermeasures and as error prevention methods. The work behind error prevention can be expensive and time consuming. The AQUIOS CL flow cytometer design has taken this into account in as many areas as possible. The AQUIOS CL flow cytometer has several systems built-in to prevent failures.
The AQUIOS CL flow cytometer has error prevention systems to assist in:
- General workflow
- Startup, cleaning, and worklist generation
- Sample preparation
- Quality control
The sections below provide an overview of each of the error prevention systems in the . . . at a Glance tables at the start of each section. Each error prevention is then explained in further detail below the table.
Error Prevention from Start to Finish on the AQUIOS CL System
The AQUIOS CL system employs several features that work to prevent errors throughout the entire workflow from start to finish. These prevention methods can be broken down into the following:
Table 1. Error Prevention from Start to Finish at a Glance
Specimen Barcodes
Unlike traditional flow cytometers, all compatible barcodes on the AQUIOS CL flow cytometer can be automatically read by the tube barcode reader without the need for a handheld barcode scanner, reducing the need to manually scan specimens. Refer to Appendix D, Barcode Specifications in the AQUIOS CL Flow Cytometer Instructions for Use (PN B21496).
Positive Specimen ID (Patient and Specimen) Monitoring
On a traditional flow cytometer, barcode scanners are used to scan both the daughter tube and the specimen into the system. This workflow has the potential for sample misidentification if an operator scans the incorrect daughter tube and/or specimen and places it in the incorrect location in the rack or carousel.
The AQUIOS CL flow cytometer reduces the possibility of sample misidentification by scanning the specimen tube right before aspiration. This form of error prevention, although new to flow cytometry, was borrowed from hematology instruments such as the DxH800.
Remote User Alerts
In current flow cytometry laboratories1, 2, an instrument operator sets up the cytometer and monitors it as the sample is run. This also implies that instruments typically have dedicated operators who need to be available to periodically monitor the instrument.
The AQUIOS CL flow cytometer monitors the sample in real time and, if three consecutive runs are flagged, it can be set up to notify the operator by a text message or an e mail. Once notified, the operator can address the issue right away. This ability allows an operator to run multiple instruments, perform other tasks in the laboratory as samples are being processed and walk away knowing that the flow cytometer can notify the operator of any significant issues. This is unique to the AQUIOS CL flow cytometer and not available on any other flow cytometer.
The AQUIOS CL flow cytometer also tracks a variety of errors throughout the workflow. Refer to Chapter 9, Troubleshooting in the AQUIOS CL Flow Cytometer Instructions for Use for a list of errors and the relevant messages tracked by the system.
Smart-Track Reagent Monitoring
Smart-Track reagent monitoring is not available on traditional sample prep devices. Traditional sample prep systems do not detect if an incorrect antibody bottle was used. Conversely, the AQUIOS system has reagents that use a unique 2D barcode identity for tracking the expiration date, on board expiration, lot, and container numbers and links this information to the sample. The reagent consumption and plate usage are monitored by the system as the samples are processed. Updates of new reagents, plate usage, reagent availability and reagent location are displayed after opening and closing the reagent door.
NOTE: The AQUIOS CL Flow Cytometer system does not track the AQUIOS Sodium Hypochlorite Solution. You must track the expiration date of the AQUIOS Sodium Hypochlorite Solution upon use.
Consumables maintained on board are automatically read into the system when placed onboard. Barcoded reagents that are not housed onboard can be read into the system by presenting the barcode on the label to the external barcode reader.
Expiration dates and reagent quantity are both measured in real time. The Reagent Status page displays the amount of remaining reagent and the number of tests remaining or the remaining reagent volume in %). Upon startup, the system displays how many tests remain before replacement is required. When a replacement is required, the system displays one of two warnings: the reagents are either low or depleted.
Scanning Control Assay Ranges
Traditional systems require that control assay ranges be input manually. This is time consuming and there is a risk of data entry errors. Since the AQUIOS CL system monitors and tracks the barcodes in real time by scanning upon loading and scanning before aspiration, samples can be loaded into the cassettes in any order and the cassettes can be loaded onto the system in any order. There is no longer a need to track a complex worklist with multiple tubes for the specimen and the daughter tube. And, there is no need to manually track the location of each daughter tube in the system. This reduces the risk of sample misidentification.
Locked System
All settings are locked except for the following: ability to adjust gates and regions* and ability to adjust compensation (not done on a regular basis). A locked system prevents user error due to erroneous setting selections or any unintended changes to the settings. A locked system makes it easy to use and allows less experienced operators to be trained to run the system.
* When adjusting regions, the system keeps a history of changes made and those changes can be undone.
Fill the form to download Workflow Comparison Study with AQUIOS CL Flow Cytometer

HIV Advanced Disease Management

HIV Advanced Disease Management Solutions
Monitoring CD4 lymphocyte counts is essential in providing critical information that impacts patient care. CD4 monitoring allows caregivers to know when the disease transforms and stage its progression so they can implement the most appropriate intervention..
Fast Throughput Subset Analysis with AQUIOS CL
In the clinical management of immune deficiency diseases, accurately counting the absolute cell numbers of leukocyte subsets and measuring the percentage of individual subtypes in blood is critical.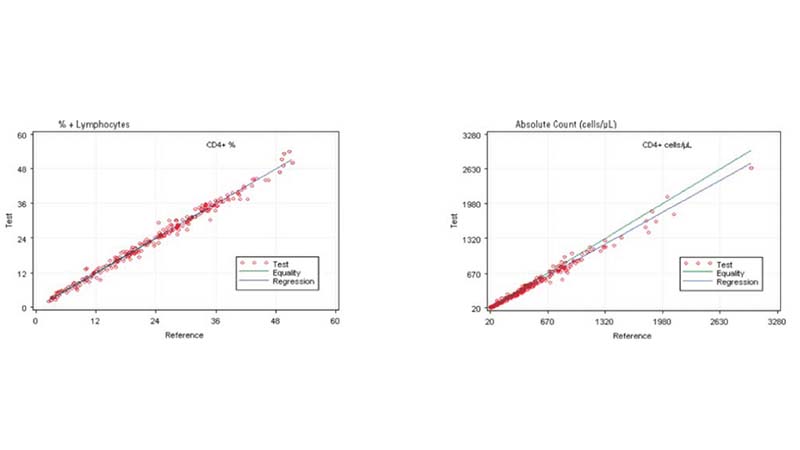
Comparing the AQUIOS CL PLG Application to the FC500 MCL FlowCARE PLG Application
Based on this study, the data suggests a strong correlation between the AQUIOS CL PLG application and the FC500 MCL FlowCARE PLG application.
AQUIOS CL Tetra and Aged Whole Blood samples
Based on this study, the AQUIOS CL instrument with Tetra application provides accurate results for recovery of the T, B and NK lymphocyte subsets in clinical and normal specimens collected into the EDTA K3 tubes when stored at room temperature for up to 24 hours.Error Prevention on AQUIOS CL overview
Laboratory standards and regulations set forth by governmental bodies such as the Centers for Disease Control (CDC) help to define the procedures that laboratories follow
Error prevention during Startup and Worklist creation
The AQUIOS CL system employs several features that work to prevent errors during startup, cleaning, and worklist generation, including Autocleaning, Test Requests to and from the LIS and Creating Worklists.
Error Prevention During Sample Prep
The AQUIOS CL system employs several features that work to prevent errors during sample prep.
Error Prevention During Quality Control
The AQUIOS CL system employs several features that work to prevent errors during quality control, including Automatically Pause when QC Fails, QC Checks, Daily QC (Controls), Real Time QC (Patient Sample).References:
- 1997 Revised Guidelines for the Performance of CD4+ T-Cell Determination in Persons with Human Immunodeficiency Virus (HIV) Infection. MMWR, January 10, 1997, p. 11.
- Levey S., Jennings, E.R., (1950) The use of control charts in the clinical laboratory. Am. J. Clin. Pathol. 20:1059.
- Hulspas R, Dombkowski D, Preffer F, Douglas D, Kildew-Shah B, Gilbert J. Flow Cytometry and the Stability of Phycoerythrin-Tandem Dye Conjugates. International Society for Advancement of Cytometry. September 23, 2009.
- Hulspas R, O’Gorman MRG, Wood BL, Gratama, JW, Sutherland DR. Considerations for the control of background fluorescence in clinical flow cytometry. Cytometry Part B 2009;76B:355-364.
- Centers for Disease Control and Prevention. 2003 Guidelines for performing single-platform absolute CD4+ T-cell determinations with CD45 gating for persons infected with Human Immunodeficiency Virus HIV). MMWR 2003; 57 (No. RR. 2): 1-13.

